Cimzia and Humira are both biologic drugs classed as tumor necrosis factor blockers or anti-TNFs (By inhibiting or stopping, TNF, these medications can reduce inflammation. That’s why they’re often called TNF inhibitors). Medications like Cimzia and Humira can help treatment of rheumatoid arthritis and other autoimmune diseases. Autoimmune diseases cannot be permanently cured and they will likely affect you for life. Cimzia and Humira aim to improve disease activity i.e. make flare-ups of symptoms happen less often and can make flare-ups less severe when they do happen. If you’re considering using one of these drugs, discussing this information with your doctor can help you decide if one of these treatments may be right for you.
What is Cimzia?
Cimzia is brand name prescription medicine, manufactured by UCB and approved by the FDA, containing the active ingredient certolizumab pegol. It is used to treat a range of inflammatory autoimmune diseases in adults, including:
- Moderately to severely active Crohn’s disease
- Moderately to severely active rheumatoid arthritis
- Moderately to severely active plaque psoriasis
- Active psoriatic arthritis
- Active non-radiographic axial spondyloarthritis with inflammation
- Active ankylosing spondylitis
What is Humira used for?
Humira is a brand-name drug containing the active ingredient adalimumab. It is approved by the FDA to treat the following inflammatory autoimmune diseases:
- Moderate to severe active rheumatoid arthritis in adults
- Moderate to severe active polyarticular (affecting multiple joints) juvenile idiopathic arthritis in children aged two years and older
- Moderate to severe chronic plaque psoriasis in adults
- Active psoriatic arthritis in adults
- Active ankylosing spondylitis in adults
- Moderate to severe active ulcerative colitis in adults
Humira is also approved to treat similar non-autoimmune inflammatory diseases:
- Moderate to severely active Crohn’s Disease in adults and children aged six years and older
- Moderate to severe hidradenitis suppurativa (HS) in adults and children aged 12 years and older
- Uveitis in adults and children aged two years and older
The main differences are that Cimzia is indicated for active non-radiographic axial spondyloarthritis with inflammation, Humira is not. Humira is indicated to treat non-autoimmune inflammatory diseases, whereas Cimzia is not. Cimzia isn’t currently approved for use in children but some indications of Humira are for use in children.
How do you take Cimzia and Humira?
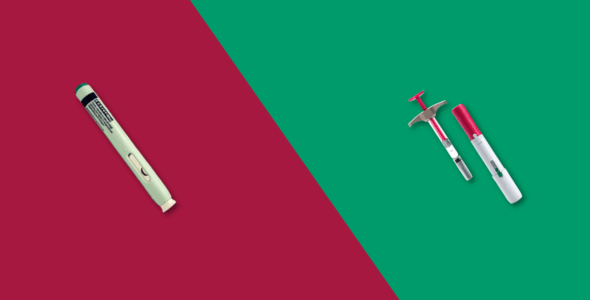
You take Cimzia and Humira by injecting it under your skin subcutaneously, either in a pre-filled syringe yourself or by injections administered by your healthcare provider. The dose of Cimzia and Humira and how many injections you need will be decided upon by your prescribing doctor based on your condition and medical history.
Cimzia comes in two forms:
- Single-dose prefilled syringe
- Single-dose vial
Humira comes in three forms:
- Single-dose injection pen
- Single-dose prefilled syringe
- Single-dose vial
How do Cimzia and Humira work?
Both drugs work as monoclonal antibodies and, more specifically as TNF blockers, to treat inflammation. TNF alpha (tumor necrosis factor-alpha) is one of a number of different antibodies produced by white blood cells and other parts of the immune system.
Once Cimzia or Humira is injected into your body, its active ingredient helps reduce inflammation. Your immune system releases a protein in your body to trigger inflammation called TNF-alpha (tumor necrosis factor-alpha). The active ingredient in Cimzia or Humira attaches to TNF-alpha and stops it from working. This limits the amount of inflammation your immune system can cause.
By reducing inflammation, Cimzia and Humira can provide relief from the symptoms of inflammatory diseases. They can also limit the damage caused by inflammatory diseases and can stop flare-ups from happening.
Does Cimzia work better than Humira?
No. The Lancet published a head-to-head clinical trial comparing Cimzia (certolizumab pegol) plus methotrexate to Humira (adalimumab) plus methotrexate in adult patients with moderate to severe rheumatoid arthritis.
The primary endpoint of the study was measured by The American College of Rheumatology (ACR) 20% improvement criteria (ACR20). The study did not show any statistically significant difference in efficacy between the two drugs.
69.2% of patients using Cimzia achieved ACR20 at 3 months vs 71.4% of patients using Humira achieved ACR20 at 3 months.
What are the side effects of Cimzia and Humira?
As Cimzia and Humira belong to the same drug class their side effects are similar.
Common side effects include:
- Injection site reactions
- Urinary tract infections
- Respiratory infections
- Abdominal pain
- Sinus infection
- Headache
- Joint pain
Serious side effects include:
- Suppression of the immune system
- Increased risk of cancer, particularly lymphoma and skin cancer
- New or worsening heart failure
- New or worsening psoriasis
- Allergic reactions
- Serious infections
- Reactivation of a hepatitis B infection if you have previously had hepatitis B
Doctors often prescribe methotrexate or corticosteroids with Cimzia and Humira increasing your risk for developing a serious infection. This is because all these drugs are immunosuppressants and reduce the ability of your body to fight infections.
What drugs interact with Cimzia and Humira?
Cimzia and Humira have drug interactions with some of the following drugs
- Live vaccines
- Anakinra (Kineret)
- Abatacept (Orencia)
- Cyclophosphamide
- Any other medications taken to treat your autoimmune disease, including Remicade (infliximab), Enbrel (etanercept), or Simponi (golimumab)
Other medical conditions may affect your treatment with Cimzia or Humira so you should always seek medical advice from the healthcare professional prescribing your drugs. The drug information provided is intended for reference only and should not be used as a substitute for medical advice.
Cimzia warning and precautions
Cimzia is not suitable for everyone. Do not take Cimzia if you:
- Are allergic to the active ingredient certolizumab pegol
- Are allergic to any of the other ingredients in Cimzia
- Are under 18 years of age
Talk to your doctor before taking Cimzia if you:
- Have or have ever had congestive heart failure
- Have or have ever had any kind of blood disorder
- Have or have ever had any condition that affects your nervous system, like multiple sclerosis or Guillain-Barré syndrome
- Often get infections, or have infections that keep coming back
- Have or have ever had hepatitis B
- Are breastfeeding or are planning to breastfeed
Humira warnings & precautions
Don’t take Humira if you:
- Are allergic to the active ingredient adalimumab
- Are allergic to any of the other ingredients found in Humira (listed in the leaflet which comes with the medication)
- Have active tuberculosis or another severe infection
- Have moderate or severe heart failure
- Are taking a medication with the active ingredients anakinra or abatacept
Humira is not recommended during pregnancy, but you may be able to breastfeed while taking it. Talk to your physician for advice.
Talk to your physician before taking Humira if you:
- Travel to regions where fungal infections are common
- Have had mild heart failure, or another serious heart condition
- Have a demyelinating disease, like multiple sclerosis
- Have the hepatitis B virus (HBV)
Can you consume alcohol while using Humira?
There is no solid scientific evidence against this, but healthcare professionals strongly advise not to consume alcohol when using Humira due to the risk of liver damage. To learn more about how Humira works and the dangers of combining it read our blog Humira and alcohol: can they be used together?
Does Cimzia cause weight gain or weight loss?
No, Cimzia doesn’t affect your weight. But if you get an infection while you’re taking Cimzia, you may lose weight. Discuss any changes in weight with your healthcare professional.
Is Cimzia available in a biosimilar?
Cimzia is not available in a biosimilar form. For more information on biosimilars read our blog What is a biosimilar drug? Biosimilar vs Generic.