Generic Symbicort Inhaler approved by FDA for asthma and COPD
Table of contents
- What is Symbicort generic inhaler?
- What does this approval mean for individuals with asthma and COPD?
- When will generic budesonide/formoterol be available?
- Are there cheaper alternatives to Symbicort available?
- How much does brand-name Symbicort cost?
- Symbicort side effects
- How do I save money on Symbicort?
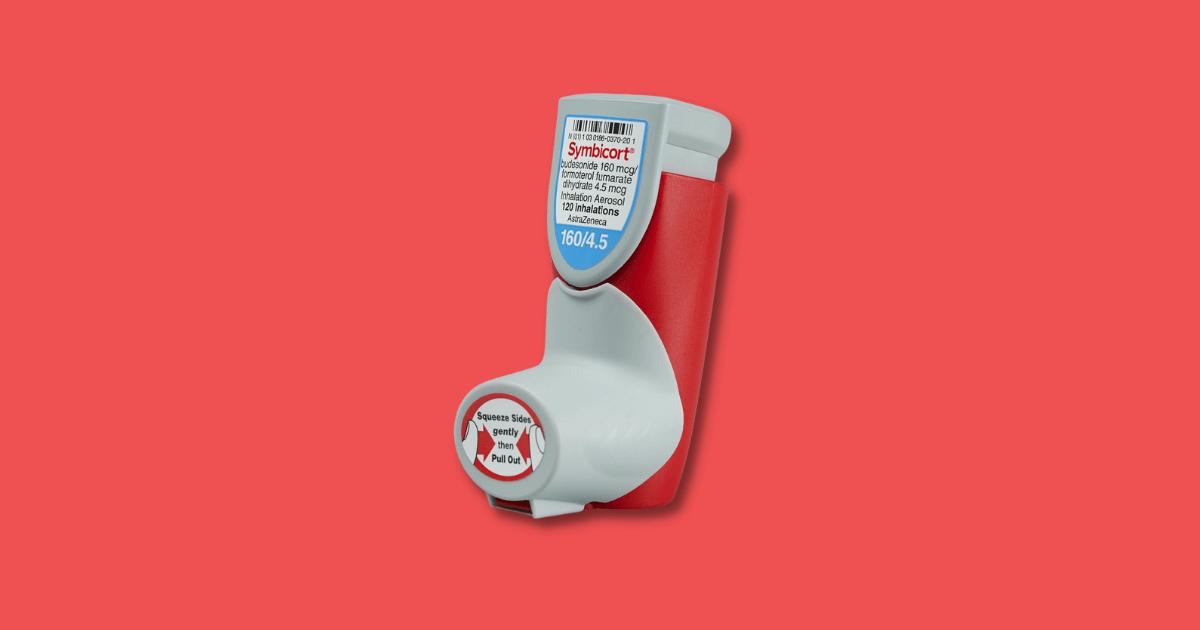
On March 15, 2022, the U.S. Food and Drug Administration (FDA) approved the first generic of Symbicort for the treatment of asthma and chronic obstructive pulmonary disease (COPD). This is great news for those who suffer from these conditions, as Generic Symbicort will be a much more affordable option than the brand name version. In this blog post, we will discuss the details of this announcement, as well as what it means for those who suffer from asthma or COPD. Asthma affects 25 million people, and more than 5 million of them are children. COPD affects more than 16 million, according to the National Heart, Lung, and Blood Institute.
Breyna will be the first generic version of Symbicort metered-dose inhaler and will be marketed and sold by Mylan Pharmaceuticals, Inc., a subsidiary of Viatris, in partnership with its drug delivery device partner Kindeva Drug Delivery LP. Mylan intends on launching Breyna in 2022 according to a press release from Viatris. According to Viatris, Breyna is a drug-device combination product, indicated for patients who are suffering from asthma or chronic obstructive pulmonary disease (COPD).
What is Symbicort generic inhaler?
Symbicort generic is a lower-cost version of the brand-name drug Symbicort and is just as effective. Similar to Symbicort, this generic version will contain a combination of an inhaled corticosteroid (budesonide) and a long-acting bronchodilator (formoterol). Symbicort is a combination of two drugs, budesonide and formoterol fumarate dihydrate. The inhaler is approved for 2 strengths: 160/4.5 mcg/actuation and 80/4.5 mcg/actuation. It is used to treat two pulmonary conditions:
- asthma in people 6 years of age and older
- maintenance treatment of airflow obstruction and reducing exacerbations for people with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema
Symbicort is a complex mixture of two drugs in a single inhaler. Budesonide is a corticosteroid that works by reducing inflammation in the lungs. Formoterol is a long-acting beta-agonist (LABA) that relaxes and opens air passages in the lungs, making it easier to breathe. It should not be used to treat acute asthma attacks.
What does this approval mean for individuals with asthma and COPD?
The U.S. Food and Drug Administration (FDA) approval of Symbicort generic means that it is just as safe and effective as the brand-name drug. This is great news for those who suffer from asthma or chronic obstructive pulmonary disease (COPD), as Symbicort generic will be a more affordable option for long-term maintenance treatment.
This FDA approval of Generic Symbicort is great news for those who suffer from asthma or COPD, as it will provide a much more affordable option. Inhalers can be very expensive, and the brand name Symbicort inhaler can cost over $300 per month. The generic version will be much cheaper, and will likely be available in late 2022.
Get your Symbicort medication for only $49 per month
Get StartedWhen will generic budesonide/formoterol be available?
The manufacturer of Breyna (budesonide and formeterol), Viatris, has indicated that Breyna should be available sometime in 2022. The exact release date for the generic version of Symbicort is unknown at this time.
The manufacturer of Symbicort, AstraZeneca, is involved in litigation with Mylan (Viatris) and depending on the outcome of the lawsuit, the generic release date may be delayed.
How much will generic Symbicort cost once it’s available?
At this time it is not known how much the generic version of Symbicort will cost. However, generic drugs are typically cheaper than brand-name drugs because they don’t have to go through the costly process of research and development.
The upcoming generic competition is great news for sufferers of asthma and COPD as the brand name Symbicort is a very expensive drug that has generated over $3.5 billion in sales for AstraZeneca for the 12 months ending in January 2021.
Are there cheaper alternatives to Symbicort available?
There are several other prescription medication alternatives to Symbicort that are available to treat asthma and COPD. These medications have different strengths, dosages, and indications.
Compare Symbicort alternatives
| Drug name | Approved uses | Side effects | Generic available |
|---|---|---|---|
| Advair Diskus (fluticasone/salmeterol) | Asthma, COPD | Hoarseness, nausea, nasopharyngitis | ✓ |
| Advair HFA (fluticasone/salmeterol) | Asthma | Hoarseness, nausea, nasopharyngitis | |
| Asmanex HFA (mometasone) | Asthma | Headache, sinusitis, nasopharyngitis | |
| Breo Ellipta (fluticasone/vilanterol) | Asthma, COPD | Headache, oral thrush, nasopharyngitis | ✓ |
| Breztri Aerosphere (budesonide/glycopyrrolate/formoterol) | COPD | Back pain, pneumonia, oral thrush | |
| Dulera (mometasone/formoterol) | Asthma | Headache, sinusitis, nasopharyngitis | |
| Flovent HFA (fluticasone) | Asthma | Sinusitis, upper respiratory infection, throat irritation | |
| Pulmicort Flexhaler (budesonide) | Asthma | Nasopharyngitis, nasal congestion, nausea | |
| Qvar Redihaler (beclomethasone) | Asthma | Nasopharyngitis, sore throat, oral thrush | |
| Singulair (montelukast) | Asthma, allergic rhinitis | Dizziness, stomach pain, headache | ✓ |
| Spiriva Respimat (tiotropium) | Asthma, COPD | Dry mouth, upper respiratory infection, sinusitis | |
| Trelegy Ellipta (fluticasone/umeclidinium/vilanterol) | Asthma, COPD | Bronchitis, nasopharyngitis, headache |
Other common alternatives to Symbicort include:
- Accolate (zafirlukast)
- Airduo RespiClick (fluticasone/salmeterol)
- Alvesco (ciclesonide)
- Anoro Ellipta (umeclidinium/vilanterol)
- Arnuity Ellipta (fluticasone)
- Asmanex Twisthaler (mometasone)
- Bevespi Aerosphere (glycopyrrolate/formoterol)
- Flovent Diskus (fluticasone)
- Incruse Ellipta (umeclidinium)
- Pulmicort respules (budesonide)
- Spiriva Handihaler (tiotropium)
- Stiolto Respimat (tiotropium/olodaterol)
- Tudorza Pressair (aclidinium)
- Wixela Inhub (fluticasone/salmeterol)
- Xolair (omalizumab)
It is recommended to obtain medical advice from your healthcare professional about any possible alternatives to Symbicort. Not all of these medications have generic alternatives available.
How much does brand-name Symbicort cost?
For people without insurance coverage for this medication, the estimated retail price for a 30-day supply of Symbicort according to the manufacturer is $303.42 (80/4.5 mcg) and $346.83 (160/4.5 mcg).
The manufacturer states that the average out-of-pocket cost for those with:
- Commercial insurance coverage is around $33.22 per month
- Medicare Part D coverage is around $31.03 per month
- Medicaid coverage is around $.90-$1.82 per month and some states offer even lower co-pays or eliminate the co-pay
Why is Symbicort so expensive?
Symbicort is a brand-name drug with no generic alternatives on the market. Typically, brand-name drugs are more expensive than generic drugs as a single pharmaceutical company has a monopoly over the sale of the medication during the patent protection period.
Symbicort side effects
Common side effects of Symbicort include:
- Throat irritation
- Thrush in the throat and mouth
- Lower respiratory tract infections
- Inflammation of the inner linings in the sinuses
- Upper respiratory tract infection
This is not a complete list of all possible side effects while using this medication. Speak with your healthcare provider if you experience any adverse reactions while using this medication.
How do I save money on Symbicort?
There are a few options to consider that could help you save money on Symbicort until the generic version is available:
- Patient assistance programs – find out if you are eligible for enrollment into the Symbicort patient assistance program.
- Ask your prescribing doctor for free samples of Symbicort – many pharmaceutical companies provide healthcare providers with free medication samples to provide to their patients to help them get started on the medication.
- Get medical advice from your healthcare professional and find out if there are any alternative treatment options – there are many Symbicort alternatives available including lower-cost generic alternatives.
- Shop around for the lowest price at multiple pharmacies – the price for Symbicort may vary by pharmacy so you should always check pricing at multiple pharmacies to find the lowest possible price for this medication.
- Symbicort coupons – check if there is a Symbicort manufacturer coupon that can help you save money when purchasing this medication.
- Get help from Medicaid – find out if you are eligible for a state Medicaid plan that may cover some or all of the cost of Symbicort.
Medically reviewed
A medical professional has reviewed this article.


Jamie Winn, PharmD
Jamie Winn, PharmD
Dr. Jamie Winn received his Doctor of Pharmacy in 2002 from the University of South Carolina College of Pharmacy, Columbia, SC. Jamie is a medical reviewer for NiceRx.